Alcohols:
What is Alcohol?
Indigenous and aboriginal tribes have been found making alcohol beverages by fermentation, thausands of years back. Fruit juices, barley, rice water, and grains were fermented and used as alcohol beverages to enjoy their religious rituals and leisure time. As time passed alcohols begun to be used as medicine but somehere in the past this practice was stopped due intoxicating property of alcohol.
So basically alcohols is a class of chemicals majorly used in the form of beverages which when ingested, it reaches to the brain causing intoxication.
Alcohols and Phenols:
Definition of Alcohols:
When -OH(hydroxyl) group is bound to hydrocarbon chain of an alkane, the new formed organic compound is called an alcohol.
If R represents an alkane, then "R-OH" is general formula of an alcohol.
Definition of Phenols:
When -OH(hydroxyl) group is bound to benzene ring, the new compound formed is called Phenol.
Molecules of alcohols and phenols are bent shaped around the oxygen atom.
Ethers:
Definition of Ethers:
When an oxygen atom is attached to two hydrocarbon chains, a new organic compound formed is called ether.
If R represents hydrocarbon chain, then the general formula of ether is "R-O-R".
Let's see some important Alcohols and Phenols:
Methanol:
Methanol also known as methyl alcohol is simplest alcohol found in many solvents and paint removers. Methanol is used in to manufacture plastics, medicines and fuels. Due to its higher octane value its used as fuel in racing cars. One needs to avoid ingestion of methanol because if ingested it gets converted to formaldehyde which cause headache, blindness and sometimes fatal.
1,2,3-Propanetriol
1,2,3- Propanetriol is populaly known as glycerol or glycerin. It is obtained as viscous liquid from fats and oils. Due to several -OH groups, glycerin is highly attracted to water and thus used majorly as a skin softener with skin lotions and cosmetics.
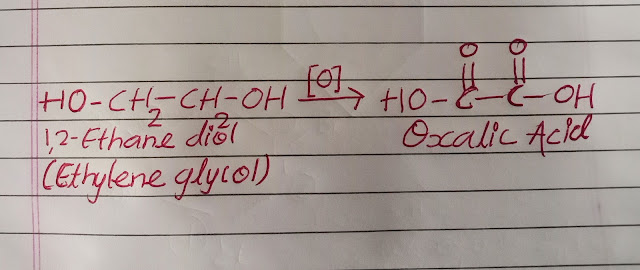
1,2-Ethanediol:
Popular known as ethylene glycol is used as solvents in paints, plastics and inks. Direct ingestion proves highly toxic as it gets converted in to oxalic acid in case if ingested which further leads to convulsions and death. It has a sweet taste which always attracts pets and children, hence proper precaution needs to be taken.
Ethylene glycol is used as an antifreeze agent in heating and cooling systems.
Phenols:
Phenols are found in Essential oils from plants such as vanilla from vanilla beans, Eugenol from cloves, isoeugenol from nutmeg, and thymol from thyme and mint.
Bisphenol A (BPA) is another phenol used in manufacturing a polycarbonate, a clear plastic that is used in making bottles.






Comments
Post a Comment