Water is the base of all life on any planet and that's why, experts look for traces of water on unknown planets, if it is or it was able to sustain any life. Planet Mars have been center of attraction since decades; And you might be aware of the efforts of the various field experts to find water in any form on Mars. Finally in 2018 scientists were successful in finding a huge subglacial lake below southern polar ice caps. Right from the morning to evening and from the birth till our death, water is an inevitable part of our life. In fact we live, eat and breathe water every day of our life time. So, the question comes why it is so important and inevitable?. Answer to these questions lies in the chemical and physical properties of water.
Lets go through the unique Chemical behavior of water molecules in three physical states:
1) In Liquid State:
- The water molecules have strong tendency to form strong Hydrogen bonds with their neighbors.
- At room temperature water molecular vibrate within the surrounding boundaries of other molecules.
- H+(proton) and OH-(hydroxy) ions tend to break apart spontaneously and move around and within the molecules.
- An average space between H+ and OH- ions is 400 molecules.
- Water is found in liquid state in normal conditions on earth and it freezes at 0 degree centigrade temperature due to strong hydrogen bonds.
- Its only substance whose liquid form is denser than the solid form.Water is at its most dense form at 4 degree centigrade temperature. Thus, ice floats on water.
- And that's why huge icebergs are formed.
- Big sheets forms on the surface of big water bodies. This insulation prevents the entire water mass below from freezing solid, which in turn allows aquatic life survive the harsh winter.
- The water molecules using intermolecular strong hydrogen bonds to form an open hexagonal structure having spaces and gaps. And hence, solid water form is less denser than the corresponding liquid form.
- Water boils at 100 degree centigrade.
- At this temperature liquid water turns into gaseous form that is water vapour.
- At this step intermolecular hydrogen bond in liquid state breaks to form a water vapor(gaseous state of water)
- This simple transformation from water to water vapor, became the driving force for the industrial revolution.
- Whatever benefits we enjoy out of industrial engineering technologies is due to this simple process.
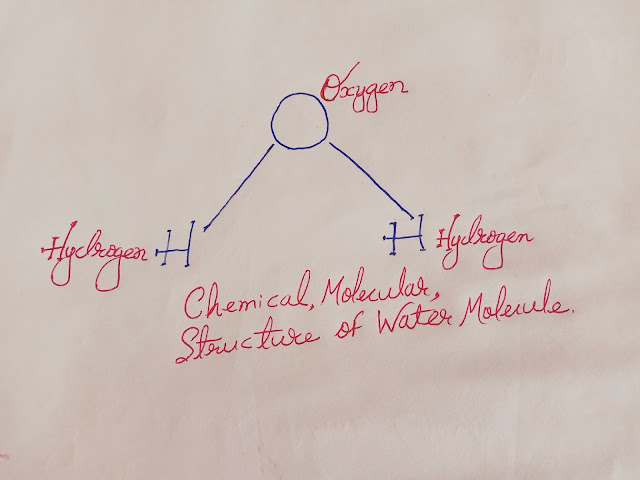
- Chemical structure of water molecule was not founded until 18th century.
- Henry Cavendish discovered hydrogen in 1766.
- Joseph Priestly discovered Oxygen in 1774.
- Renowned english chemist Henry Cavendish found that, when measured quantity of hydrogen and oxygen are burnt, the only result is water.
- This was further confirmed by the French chemist Antoine Lavoisier,when he decomposed water back to hydrogen and oxygen.
- Finally in 1871, the world-famous chemist Stanislao Cannizaro, established the formulae of water to be H2O.
Thanks for reading my blog. Guy's I will love to hear from you. Your every feedback will help me give you a better scientific content each time.

Comments
Post a Comment